
United States Nuclear Regulator Committee.
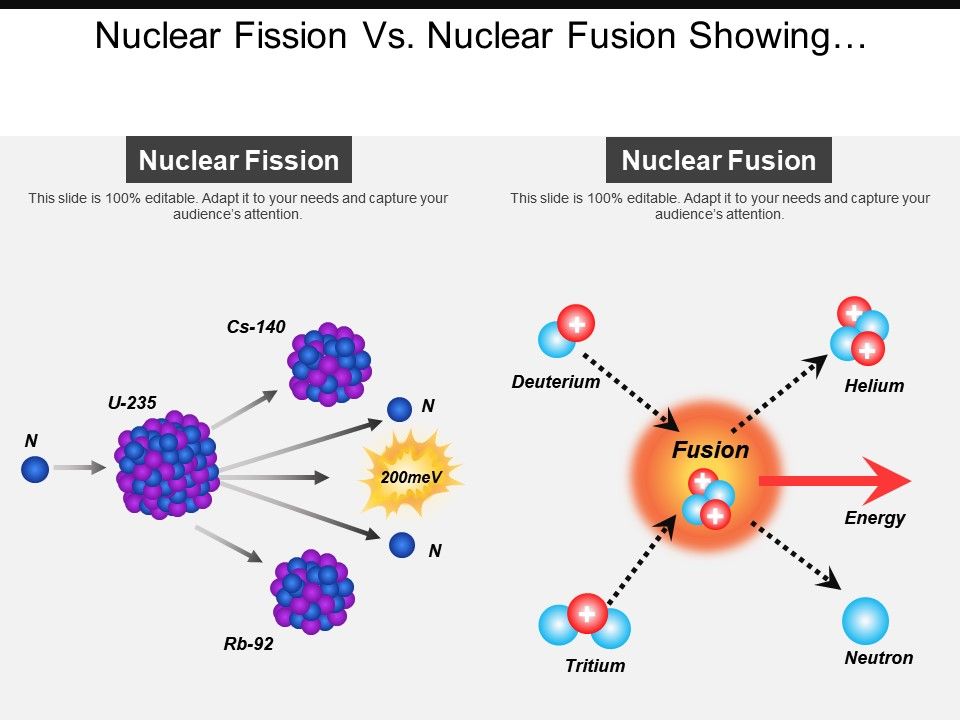
Nuclear fusion is more widely known for its usage in something like the hydrogen bomb where different isotopes fuse together and release a massive amount of energy (17.6 MeV to be exact)(Atomicarchive, 2015, Para 1). An example of nuclear fusion would be the power that fuels both the sun and the stars. Nuclear fusion is simply explained as the process in which the fusion of two “light elements”, which are elements with smaller atomic numbers, release nuclear energy. This is no longer an option because they can no longer operate and are in the process of decommissioning. Nuclear power relies on fissionable material that can sustain a chain reaction with neutrons.Examples of such materials include uranium and plutonium. There is also a known 3rd method called “Heap Leaching” that has been used to extract the uranium from ore at the conventional mills. How long will global uranium deposits fuel the world's nuclear reactors at present consumption rates Steve Fetter, dean of the University of Maryland's School of Public Policy, supplies an answer. Uranium occurs in combination with small amounts of other elements. Uranium is a common metal found in rocks all over the world. control rods between the fuel rods in the reactor to absorb excess neutrons. Fast breeder reactors uses also fuels rich in fissile nuclei.However, the mining method of obtaining uranium isn’t used very much anymore as there is a method which instead of mining, they inject chemicals into underground uranium deposits to dissolve or “leach” uranium from the ore. Nuclear explained Where our uranium comes from Basics +Menu The United States imports most of the uranium it uses as fuel Uranium is the fuel most widely used by nuclear power plants for nuclear fission. When a nucleus of uranium-235 undergoes fission, it splits into two smaller. The core of these embarked reactors is compact. They serve to propel submarines, aircraft carriers and icebreakers, ensuring their autonomy. Some specific reactors operate with a fuel containing a much higher proportion of fissile nuclei than the commercial reactors producing electricity. The MOX fuel is used in some French PWR reactors tranformed to accept this fuel. The reprocessing of the spent fuel in France at La Hague plant allows to separate plutonium and to mix plutonium, which is fissile, with uranium to produce a mixed fuel, called MOX. After reprocessing the spent fuel, it is possible enrich again this uranium in order to recycle it as a fresh fuel. The uranium that comes out is still richer in the isotope 235 than natural uranium. The production of electrical energy by nuclear fission is widespread, while nuclear fusion is still under development. The spent fuel must be unloaded from the reactor core after three to four years. These reactions produce heat that is then used to generate electricity. Minor actinides, nuclei heavier than uranium, accumulate, as well as fission products. Nuclear energy is energy created via a fission or fusion reaction of atoms. A fraction of the fissile uranium-235 is replaced by plutonium-239 which is also fissile. Uranium is now used to power commercial nuclear reactors that produce electricity and to produce isotopes used for medical, industrial, and defense purposes.

The fuel is depleted, the operating conditions vary. Once introduced into the reactor, the fuel composition changes. Most reactors today use a degree of enrichment between 3 and 4.5%. Because EM2 uses more of its uranium fuel and converts it more efficiently to energy, it can operate up to 30 years on the same load of fuel. For the sustainability of chain reaction, this number should be significantly higher than 1 to compensate for the various losses: this condition holds comfortably with fuel enriched to 3.5% as that of PWRs but marginally with natural uranium which contains only 0.7% uranium-235. The number of secondary neutrons produced on average by slow neutron capture in uranium nuclei increases with the ratio of the fissile uranium-235 isotope. It is easier to run a reactor loaded with enriched uranium than with natural uranium. First, it creates radioactive isotopes lighter than uranium, namely cesium-137 and strontium-90, which generate most of the heat and penetrating radiation in the spent fuel. The nuclear fission process results in two different kinds of radioactive waste in spent fuel. Neutrons available for fission in uranium fuels SNFs extreme radioactivity declines very quickly.


 0 kommentar(er)
0 kommentar(er)
